Diagnostic Accuracy of JES Classification for Prediction of Invasion Depth in Superficial Esophageal Squamous Carcinoma: Meta-Analysis
By Dou Fu1, Hui Zhao2, Wenquan Lan2, Yuanfa Li2Affiliations
doi: 10.29271/jcpsp.2024.03.336ABSTRACT
Prediction of the depth of invasion in superficial oesophageal squamous carcinoma (SESC) is an important factor for choosing the treatment. Recently, the Japan Esophageal Society (JES) designed a magnifying endoscopy classification based on the Inoue and Arima classifications. The aim of this study was to conduct a meta-analysis of the published literature on JES classification. Meta-Disc version 1.4, Review Manager 5.4 as well as stata 14.0 were used. The analysis combined sensitivity and specificity with the respective 95% CI, to draw a summary receiver operating characteristic curve (SROC), and estimated the area under curve (AUC) for overdiagnosis and underdiagnosis for each type B. Eight studies were included in the meta-analysis comprising 1279 patients. Type B1 has high sensitivity (0.86, 95%CI: 0.83-0.88) and specificity (0.84, 95%CI: 0.81-0.88) for the prediction of EP/LPM classifications. The AUC was calculated to be 0.92 with a high proportion of underdiagnosis (17%). The sensitivity and specificity of type B2 were 0.66 (95% CI, 0.6-0.72) and 0.84 (95% CI, 0.82-0.86) respectively. The overdiagnosis and underdiagnosis of type B2 were 14% and 39%. Type B3, sensitivity was low (0.49, 95% CI: 0.41-0.56), with high specificity and AUC (specificity: 0.99; AUC: 0.95).
JES classification is a useful and reliable modality for predicting the depth of invasion of SESC, but other modalities should be considered for additional assessment when type B2 is detected.
Key Words: JES classification, Superficial oesophageal squamous carcinoma, Depth of invasion, Magnifying endoscopy, Meta-analysis.
INTRODUCTION
Endoscopic resection (ER), one of the standard treatments, has been gradually used worldwide for superficial oesopha-geal squamous carcinoma (SESC).1-3 Although ER is a minimally invasive treatment for SESC, this endoscopic procedure crucially depends on the tumour invasion depth which is directly related to lymph node metastasis.4,5 Hence, effective prediction of invasion depth in SESC is important for choosing a suitable treatment method.
Magnifying endoscopy (ME) is one of the most used modalities (e.g. non-magnified endoscopy and endoscopic ultrasound) for the prediction of invasion depth of SESC. ME offers clear screening of intrapapillary capillary loops (IPCLs) of the oesophageal mucosa and ME combined with narrow-band imaging (ME-NBI) could further provide enhanced observation of the microvascular architecture, which is closely associated with the invasion depth in SESC.6-8
Until now, there were three ME classifications designed by experts in Japan and the Japan Esophageal Society (JES) for predicting the depth of invasion in SESC.8-10 However, among the three ME classifications, the first two classifications are difficult to use due to their complicated items.8,9 In 2016, the JES demonstrated that a new ME classification based on types of IPCLs is easy to understand and effective for predicting the depth of invasion of SESC.10
Although some meta-analyses and systematic reviews were published for evaluating the diagnosing accuracy of invasion depth of SESC previously, there was no meta-analysis focusing on the new ME classification.11-13 Because pooled outcomes based on different ME classifications could result in high heterogeneity and bias. So, this the meta-analysis and systematic review was designed to evaluate the diagnostic accuracy of the JES classification for invasion depth of SESC.
METHODOLOGY
The study was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) statement of DTA Studies.14 The search of databases included Embase, PubMed, and Cochrane Library. The search items used were “superficial oesophageal squamous carcinoma” OR “superficial oesophageal squamous cell carcinoma” and “magnifying endoscopy”, covering the period from the establishment of each database to August 2023; the search language was English. The inclusion criteria for the study were a JES classification for diagnosis of depth of invasion in SESC; complete data (2*2 contingency table); and the gold standard was a pathological diagnosis. Reviews, case reports, and studies with incomplete data were excluded. The first and the last authors extracted the data from the included studies and evaluated the quality of the included studies independently. The Quality Assessment of Diagnostic Accuracy Studies- 2 (QUADAS-2) tool was used to assess the quality.15 The primary outcome considered was the accuracy of type B for predicting depth of invasion in SESC, and the secondary outcome indicators were overdiagnosis and underdiagnosis which were defined as overdiagnosis and underdiagnosis proportion in each type B group (i.e. B1, B2, and B3) (13) Meta-Disc version 1.4 was used to perform heterogeneity and the diagnostic accuracy of JES classification, the latter of which was calculated by pooled estimates of sensitivity, specificity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), and diagnostic odds ratio (DOR) with corresponding 95% confidence intervals (CIs). Summary receiver-operating characteristic (SROC) curves, which estimate the area under the curve (AUC), were also calculated. Heterogeneity was evaluated by I2 index, with an I2> 50% suggesting high heterogeneity. Pooled estimates were calculated by using the random effects model (REM). Review Manager 5.4 was used for the presentation of quality assessment. Deeks funnel were used to assess publication bias and calculated the overdiagnosis and underdiagnosis by using STATA software (version 14).
RESULTS
Two hundred and seventy-eight papers were found from PubMed, Embase, and Cochrane library. Eight studies were finally included after excluding duplicates, reviews, descriptive studies and studies that did not meet the inclusion criteria Figure 1.10,16-22
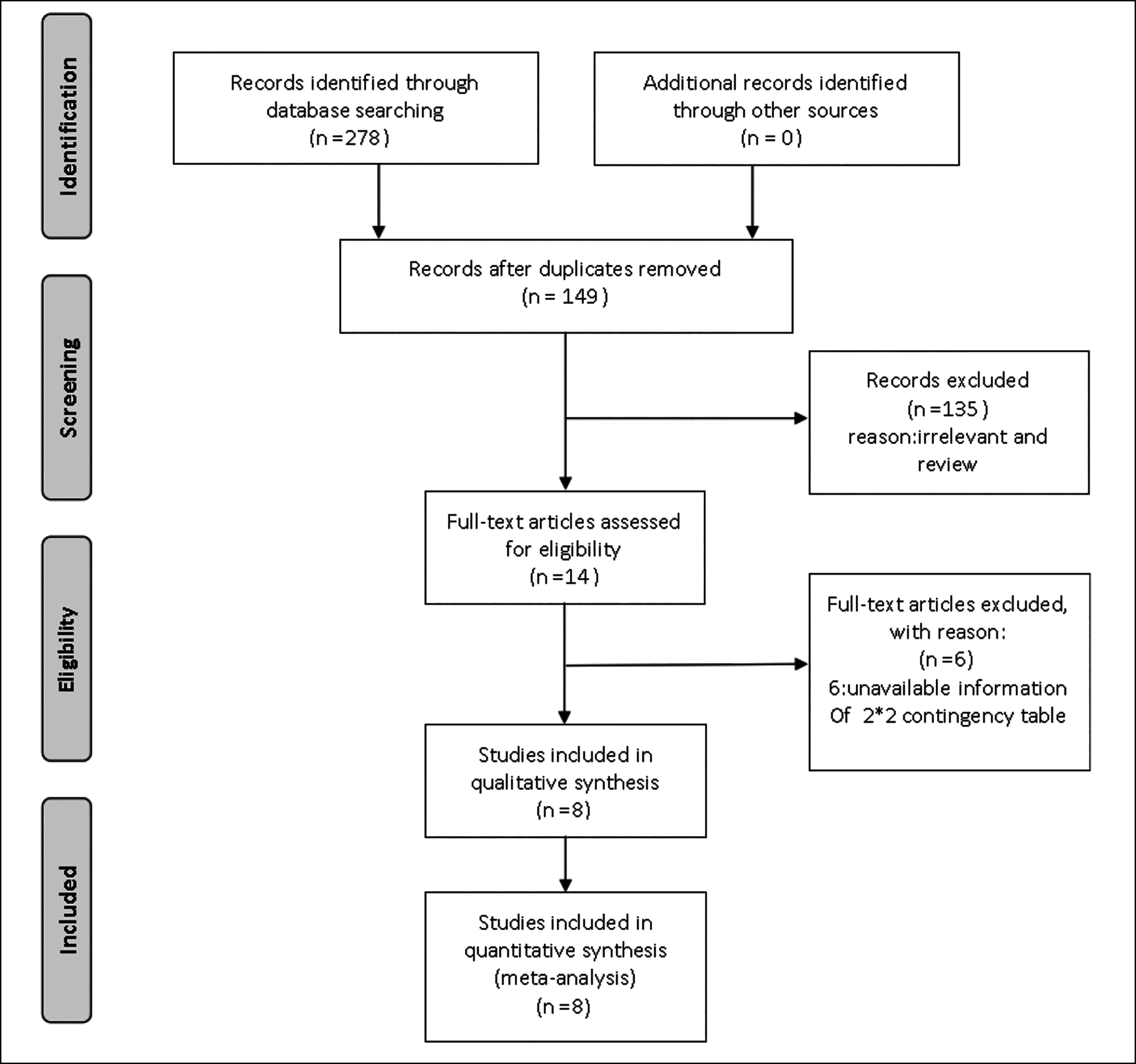 Figure 1: PRISMA flow diagram.
Figure 1: PRISMA flow diagram.
Eight articles included 1279 patients with 1329 SESC lesions, ageing from 45 to 91 years. Among the eight studies, seven were retrospective studies and one was prospective study (Table I). Since seven studies, only included the patients who underwent ER, while patients who underwent surgery or radiotherapy were not included, there was a high risk of bias in patient selection. All studies did not report the timing between ME procedure and pathological assessment, this may result in bias (Figure 2).
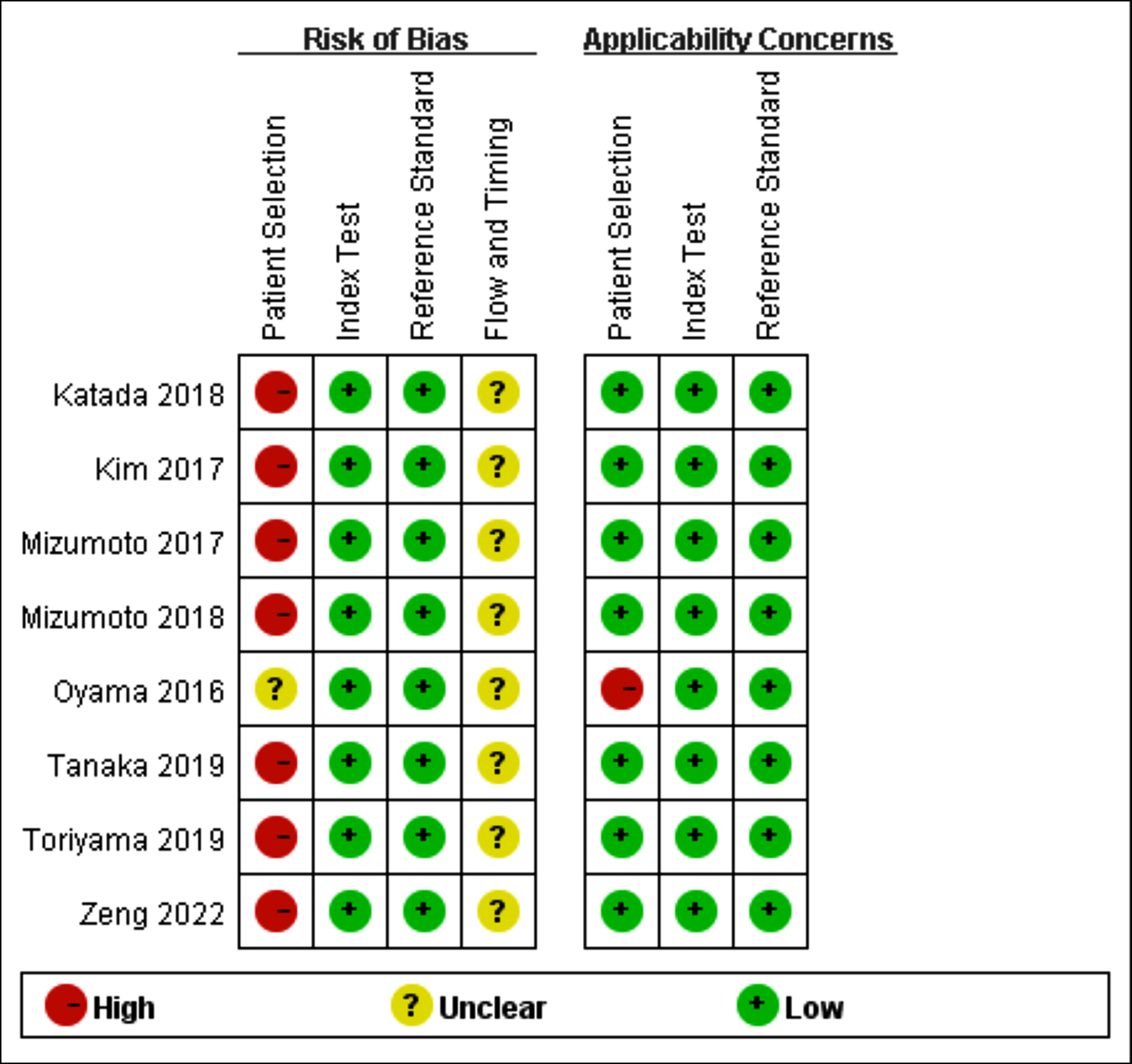 Figure 2: Quality assessment summary of each study.
Figure 2: Quality assessment summary of each study.
The Spearman correlation coefficients of ME-NBI for type B1, B2, and B3 were 0.405 (p= 0.32), 0.619 (p= 0.102), and 0.31(p= 0.456), respectively, which indicated the absence of a diagnostic threshold effect.
Regarding type B1 for prediction of epithelium or lamina propria mucosae (EP/LPM), the sensitivity (I2= 85.2%) and specificity (I2= 73.1%) also reflected significant heterogeneity, with a pooled sensitivity of 0.86 (95% CI: 0.83–0.88) and a pooled specificity of 0.84 (95% CI: 0.81–0.88). The SROC with pooled AUC was 0.92. The summary PLR and NLR were 4.9 (95% CI: 3.61–6.67) and 0.17 (95% CI: 0.12–0.25), respectively (Figure 3). The DOR was 35.13 (95% CI: 20.07–61.51) and the underdiagnosis rate was (0.170.07-0.28). Compared with type B1, the pooled sensitivities of type B2 and type B3 were lower and with more overdiagnosis and underdiagnosis rates. The diagnostic accuracy of type B2 and B3 was shown in Table II and Figure 4 and 5.
Univariate meta-regression analysis was performed to test the relation between diagnostic accuracy and the following variables: design type, country, and endoscope. However, no variable was found to be responsible for the significant heterogeneity. Deeks funnel plot of studies for each type B exhibited a symmetrical shape for the regression line and found no evidence of publication bias.
Table I: Characteristics of the included studies.
|
Author, year |
Country |
Study design |
Patients |
Lesions |
Male (%) |
Age |
Histopathology |
Endoscope |
|
Oyama, 2016 |
Japan |
Prospective, multicentre study |
211 |
211 |
NA |
NA |
SESCC |
GIF-H260Z or GIFQ240Z |
|
Mizumoto, 2017 |
Japan |
Retrospective, single-centre |
174 |
174 |
153 (87.9%) |
67.7 ± 8.8 |
SESCC |
GIF-H260Z or GIFQ240Z or H290Z |
|
Kim, |
Korea |
Retrospective, muti-centre |
69 |
70 |
62 |
66 (47-81) |
SESCC |
GIF-H260Z |
|
Katada, 2018 |
Japan |
Retrospective, single-centre |
256 |
256 |
224 (87.5%) |
71 (46-91) |
SESCC |
GIF-H260Z or GIFQ240Z |
|
Mizumoto, 2018 |
Japan |
Retrospective, single-centre |
136 |
136 |
112 (82.4%) |
68.2±8.9 |
SESCC |
H260Z or H290Z |
|
Tanaka, 2019 |
Japan |
Retrospective, single-centre |
207 |
248 |
168 (81.2%) |
70.2 (45-89) |
SESCC |
GIF-H260Z |
|
Toriyama, 2019 |
Japan |
Retrospective, single-centre |
82 |
82 |
63 |
64.7 ± 9.06 |
SESCC |
NA |
|
Zeng, |
China |
Retrospective, single-centre |
144 |
152 |
198 |
61.3 ± 7.5 |
SESCC |
GIF-H260Z or EG-L590ZW |
|
SESCC: Superficial oesophageal squamous cell carcinoma; NA: No available. |
||||||||
Table II: Diagnostic accuracy of each Type B.
|
Subtypes |
Prediction of invasion depth |
Sensitivity |
Specificity |
Positive likelihood ratio |
Negative likelihood ratio |
Diagnostic odds ratio |
AUC |
Overdiagnosis |
Underdiagnosis |
|
Type B1 |
EP/LPM |
0.86 (0.83-0.88) |
0.84 (0.81-0.88) |
4.90 (3.61-6.67) |
0.17 (0.12-0.25) |
35.13 (20.07-61.51) |
0.92 |
NA |
0.17 (0.07-0.28) |
|
Type B2 |
MM/SM1 |
0.66 (0.6-0.72) |
0.84 (0.82-0.86) |
4.79 (3.39-6.76) |
0.34 (0.2-0.57) |
16.84 (10.1-28) |
0.87 |
0.14 (0.07-0.21) |
0.39 (0.25-0.52) |
|
Type B3 |
SM2 |
0.49 (0.41-0.56) |
0.99 (0.98-0.99) |
28.4 (16.48-48.94) |
0.55 (0.44-0.69) |
57.11 (30.25-107.8) |
0.95 |
0.01 (0-0.02) |
NA |
|
CI: Confidence interval; AUC: Area under curve. |
|||||||||
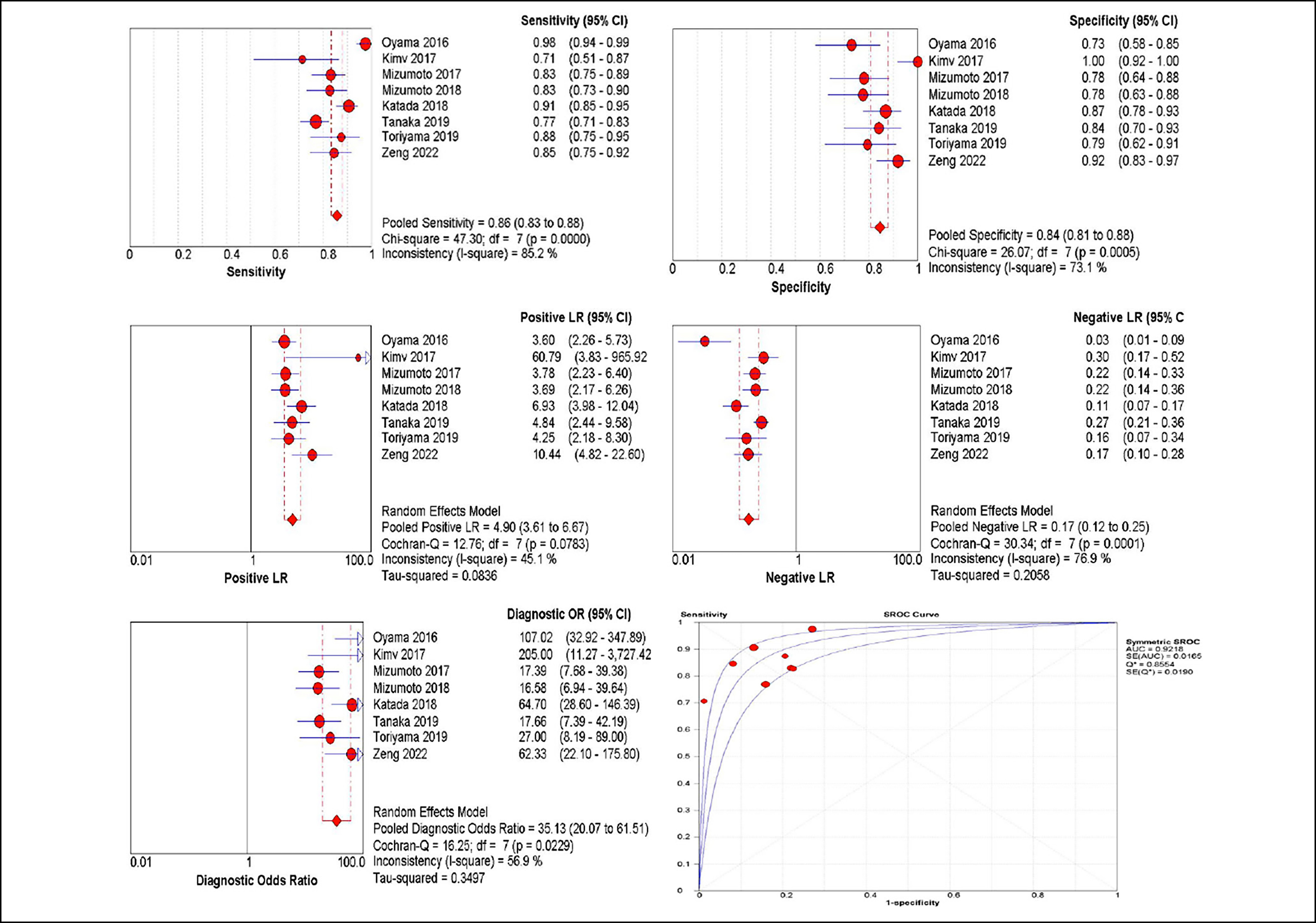 Figure 3: Diagnostic performance of Type B1.
Figure 3: Diagnostic performance of Type B1.
 Figure 4: Diagnostic performance of Type B2.
Figure 4: Diagnostic performance of Type B2.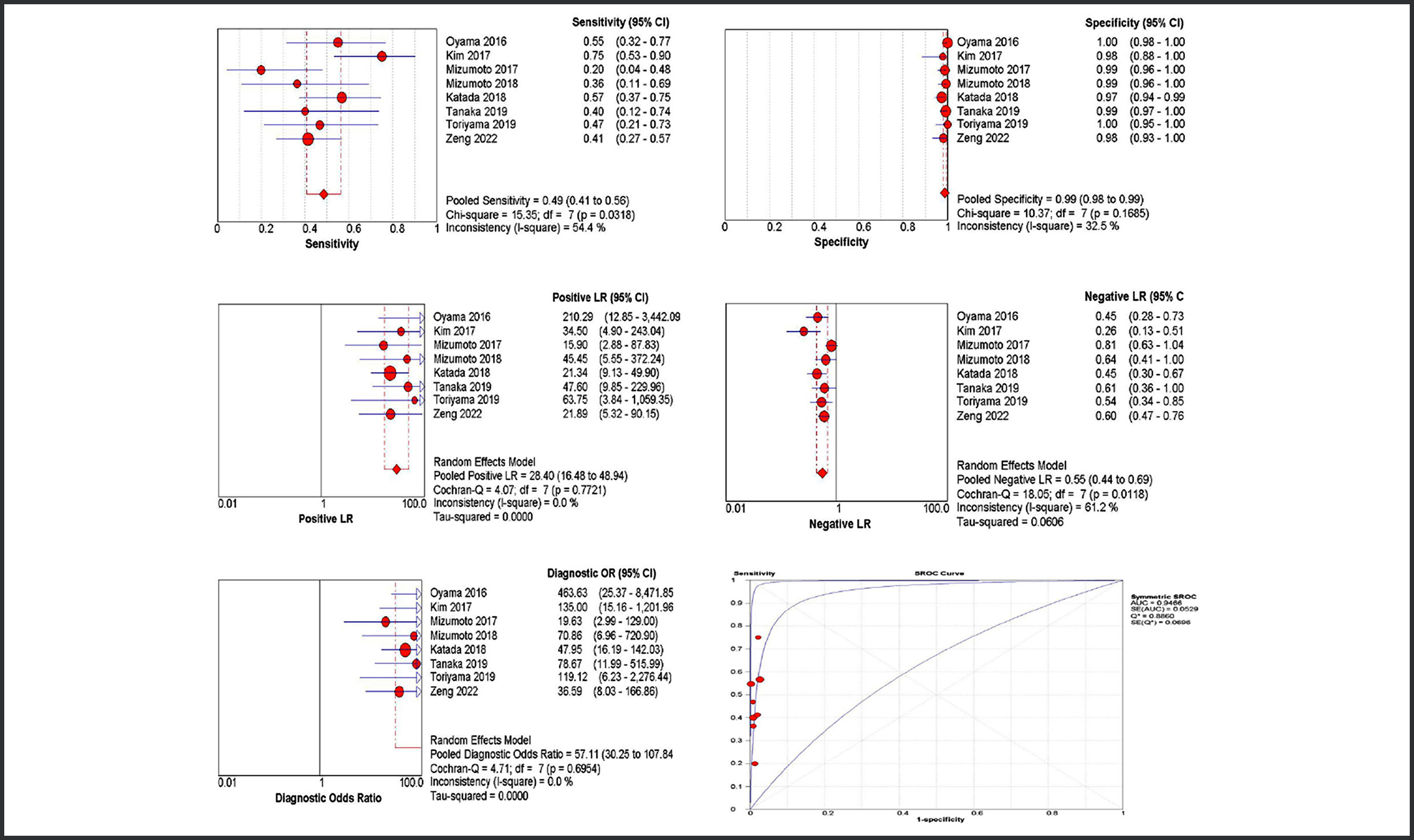 Figure 5: Diagnostic performance of Type B3.
Figure 5: Diagnostic performance of Type B3.
DISCUSSION
Predicting the depth of ESCC invasion is important before the treatment because lymph node (LN) metastasis is rarely observed in these lesions.23 ER is an effective therapeutic method for ESCC in EP/LPM stage.24 The review is the first meta-analysis to focus on the diagnostic accuracy of the JES classification for invasion depth of SESC and calculate the pooled estimates of each type B IPCLs. The JES classification is useful for predicting the depth of invasion. The accuracy of type B1 for the prediction of EP/LPM and B3 for the prediction of SM2 was high, but the accuracy of type B2 for MM/SM1 was moderate with high underdiagnosis (39%). The deficiency of type B2 may be assisted by other modalities (i.e. an avascular area) in order to improve the diagnosis of the invasion depth of ESCC.25
Regarding type B1 for the prediction of EP/LPM, JES classification showed a strong power with an AUC of 0.92. The other diagnostic parameters (i.e. sensitivity, specificity and NLR) of type B1 indicate that JES classification is a reliable modality for confirming deep cancer invasion of EP/LPM. The results were consistent with previous reports. Although a high proportion of underdiagnosis in type B1 was found, most cases of underdiagnosis were from MM/SM1 and ER was suitable for both lesion infiltration depths.
In terms of Type B2 and type B3, JES classification showed poor sensitivities (0.66 in type B2 and 0.49 in type B3). The pooled estimates showed type B2 with moderate AUC (0.87) and a high rate of underdiagnosis (39%). This may result in unnecessary ER in some ESCC patients, whereas surgery could be more necessary. Although strong evidence does not exist, the prediction of the depth of tumour invasion may be improved by the additional utility of NBI magnifying imaging (i.e. an vascular area) or EUS before selecting therapeutic strategies.16,26 The specificity of type B3 was very high (0.99) and with high AUC (0.96). This indicates that type B3 is accurate predictor of SESC in SM2. However, the sensitivity of type B3 for SM2 was lower (0.49). Interestingly, endoscopic ultrasonography with a high-frequency miniature probe was reported to supplement the low sensitivity of type B3.22 Combining ME and EUS enables the most comprehensive assessment of submucosa infiltration depth.
Compared with previous meta-analyses and systematic reviews, the study is the first meta-analysis and systematic review focusing on JES classification.11-13 In addition, the authors excluded the studies including the Arima classification and Inoue classification in order to decrease the bias. Similar to the results of previous meta-analyses on magnified endoscopy, JES classification based on changes of vascular patterns on the mucosa of the oesophagus is useful for the diagnosis of cancer invasion depth.
There were some limitations in this study. First of all, heterogeneity was high and the source was not found among the analysis. It was speculated that the heterogeneity may be related to the different experiences of endoscopists. Secondly, most studies were retrospectively performed. Third, most included studies enrolled patients who underwent ER but not surgery. This affected the sample size and patient selection. Therefore, these limitations bring high heterogeneity and some potential bias and indicate that prospective studies containing similar experienced endoscopists need to be performed to confirm this issue.
CONCLUSION
JES classification is a useful and reliable modality for predict-ing depth of invasion of SESC, and other modalities should be considered when type B2 exists.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
DF, YL: Design, acquisition and analysis of data, and writing of the manuscript.
DF, HZ, WL: Interpretation and discussion of results.
YL: Revision of the manuscript.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Berger A, Rahmi G, Perrod G, Pioche M, Canard JM, Cesbron-Métivier E, et al. Long-term follow-up after endoscopic resection for superficial esophageal squamous cell carcinoma: a multicenter western study. Endoscopy 2019; 51(4): 298-306. doi: 10.1055/a-0732-5317.
- Pimentel-Nunes P, Libânio D, Bastiaansen BAJ, Bhandari P, Bisschops R, Bourke MJ, et al. Endoscopic submucosal dissection for superficial gastrointestinal lesions: European Society of Gastrointestinal Endoscopy (ESGE) Guideline - Update 2022. Endoscopy 2022; 54(6): 591-622. doi: 10. 1055/a-1811-7025.
- Ishido K, Tanabe S, Katada C, Kubota Y, Furue Y, Wada T, et al. Usefulness of endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma in elderly patients: a single-center retrospective cohort study. .Jpn J Clin Oncol 2021 28; 51(6):895-904. doi: 10.1093/jjco/hyab 030.
- Moon JY, Kim GH, Kim JH, Kim HH, Ryu KD, Park SO, et al. Clinicopathologic factors predicting lymph node metastasis in superficial esophageal squamous cell carcinoma. Scand J Gastroenterol 2014; 49(5): 589-94. doi: 10.3109/00365 521.2013.838604.
- Eguchi T, Nakanishi Y, Shimoda T, Iwasaki M, Igaki H, Tachimori Y, et al. Histopathological criteria for additional treatment after endoscopic mucosal resection for esophageal cancer: analysis of 464 surgically resected cases. Mod Pathol 2006; 19(3):475-80. doi: 10.1038/modpathol.38 00557.
- Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE, et al. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc 2004; 59(2):288-95. doi: 10.1016/s0016- 5107(03)02532-x.
- Santi EG, Inoue H, Ikeda H, Yoshida A, Onimaru M, Sato H, et al. Microvascular caliber changes in intramucosal and submucosally invasive esophageal cancer. Endoscopy 2013; 45(7):585-8. doi: 10.1055/s-0033-1344228.
- Sato H, Inoue H, Ikeda H, Sato C, Onimaru M, Hayee B, et al. Utility of intrapapillary capillary loops seen on magnifying narrow-band imaging in estimating invasive depth of esophageal squamous cell carcinoma. Endoscopy 2015; 47(2):122-8. doi: 10.1055/s-0034-1390 858.
- Arima M, Tada T, Arima H. Evaluation of microvascular patterns of superficial esophageal cancers by magnifying endoscopy. Esophagus 2005; 2(4):191-7. doi:10. 1007/s10388-005-0060-6.
- Oyama T, Inoue H, Arima M, Momma K, Omori T, Ishihara R, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esopha-geal Society. Esophagus 2017; 14(2): 105-12. doi: 10.1007/ s10388-016-0527-7
- Ishihara R, Matsuura N, Hanaoka N, Yamamoto S, Akasaka T, Takeuchi Y, et al. Endoscopic imaging modalities for diagnosing invasion depth of superficial esopha-geal squamous cell carcinoma: a systematic review and meta-analysis. BMC Gastroenterol 2017; 17(1):24. doi: 10.1186/s128 76-017- 0574-0.
- Yu T, Geng J, Song W, Jiang Z. Diagnostic Accuracy of Magnifying Endoscopy with Narrow Band Imaging and Its Diagnostic Value for Invasion Depth Staging in Esophageal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Biomed Res Int 2018; 2018:8591387. doi: 10.1155/ 2018/8591387.
- Inoue T, Ishihara R, Shibata T, Suzuki K, Kitagawa Y, Miyazaki T, et al. Endoscopic imaging modalities for diagnosing the invasion depth of superficial esophageal squamous cell carcinoma: A systematic review. Esophagus 2022; 19(3):375-83. doi: 10.1007/s10388-022- 00918-5.
- McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM and the PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA State-ment. JAMA 2018; 319(4):388-96. doi: 10.1001/jama.2017. 19163.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155(8): 529-36. doi: 10.7326/0003- 4819-155- 8-201110180-00009.
- Mizumoto T, Hiyama T, Oka S, Yorita N, Kuroki K, Kurihara M, et al. Diagnosis of superficial esophageal squamous cell carcinoma invasion depth before endoscopic submucosal dissection. Dis Esophagus 2018; 31(7):1-7. doi: 10.1093/dote/dox142.
- Kim SJ, Kim GH, Lee MW, Jeon HK, Baek DH, Lee BE, et al. New magnifying endoscopic classification for superficial esophageal squamous cell carcinoma. World J Gastroenterol 2017; 23(24):4416-21. doi: 10.3748/wjg. v23.i24.4416.
- Katada C, Tanabe S, Wada T, Ishido K, Yano T, Furue Y, et al. Retrospective assessment of the diagnostic accuracy of the depth of invasion by narrow band imaging magnifying endoscopy in patients with superficial esophageal squamous cell carcinoma. J Gastrointest Cancer 2019; 50(2): 292-7. doi: 10.1007/s12029-018- 0075-6.
- Mizumoto T, Hiyama T, Quach DT, Sanomura Y, Urabe Y, Oka S, et al. Magnifying endoscopy with narrow band imaging in estimating the invasion depth of superficial esophageal squamous cell carcinomas. Digestion 2018; 98(4):249-56. doi: 10.1159/000489490.
- Tanaka I, Hirasawa D, Saito H, Matsuda T, Nakahori M, Maeda Y, et al. The sub-classification of type B2 vessels according to the magnifying endoscopic classification of the Japan Esophageal Society. Dig Endosc 2020; 32(1):49-55. doi: 10.1111/den.13459.
- Toriyama K, Tajika M, Tanaka T, Ishihara M, Hirayama Y, Onishi S, et al. Clinical relevance of fluorodeoxyglucose positron emission tomography/computed tomography and magnifying endoscopy with narrow band imaging in decision-making regarding the treatment strategy for esophageal squamous cell carcinoma. World J Gastroenterol 2019; 25(46):6767-80. doi: 10.3748/wjg.v25.i46. 6767.
- Zeng YT, Sun YY, Tan WC, Luo SA, Zou BH, Luo GY, et al. Study of preoperative diagnostic modalities in Chinese patients with superficial esophageal squamous cell carcinoma. World J Gastrointest Surg 2022; 14(9):986-96. doi: 10.4240/wjgs.v14.i9.986.
- Miwata T, Oka S, Tanaka S, Kagemoto K, Sanomura Y, Urabe Y, et al. Risk factors for esophageal stenosis after entire circumfer ential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc 2016; 30:4049-56. doi: 10.1007/ s00464-015-47 19-3.
- Kuwano H, Nishimura Y, Oyama T, Kato H, Kitagawa Y, Kusano M, et al. Guidelines for diagnosis and treatment of carcinoma of the esophagus April 2012 edited by the Japan esophageal society. Esophagus 2015; 12(9): 1-30. doi: 10.1007/s10388-014-0465-1.
- Gotoda T, Hori K, Nakagawa M, Kobayashi S, Toyokawa T, Ishiyama S, et al. A prospective multicenter study of the magnifying endoscopic evaluation of the invasion depth of superficial esophageal cancers. Surg Endosc 2022; 36(5): 3451-59. doi: 10.1007/s00464-021-08666-w.
- Lee MW, Kim GH, I H, Park DY, Baek DH, Lee BE, et al. Predicting the invasion depth of esophageal squamous cell carcinoma: comparison of endoscopic ultrasonography and magnifying endoscopy. Scand J Gastroenterol 2014; 49(7): 853-61. doi: 10.3109/00365521.2014.91 5052.